TCT 2025: Transcatheter or Surgical Aortic-Valve Replacement in Low-Risk Patients at 7 Years, PARTNER-3 Trial Findings
- byDoctor News Daily Team
- 29 October, 2025
- 0 Comments
- 0 Mins
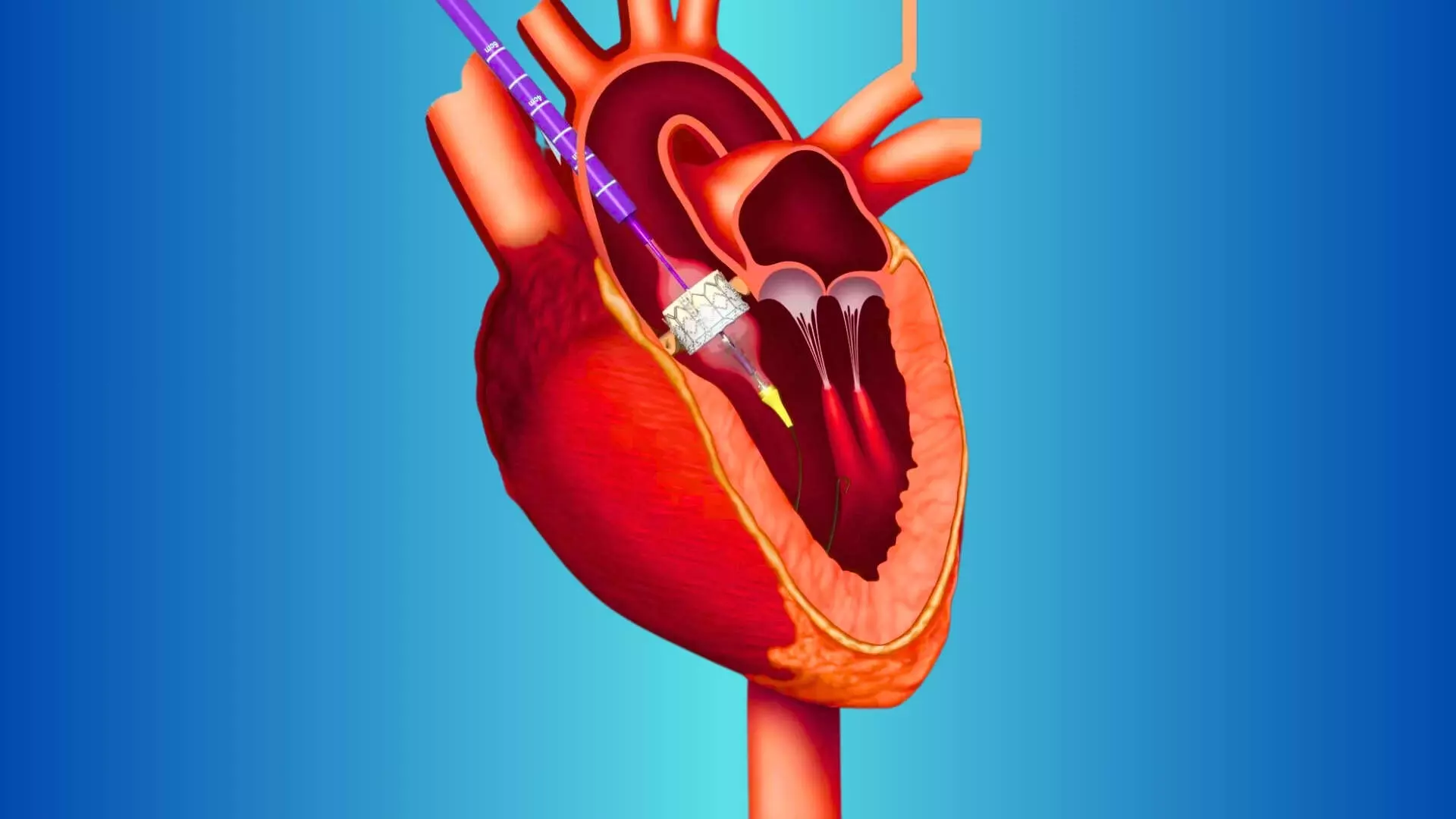
This news is covered by the Bureau present at the TCT Conference 2025, being held in San Francisco, USA. At seven years of follow-up, transcatheter aortic valve replacement (TAVR) with the SAPIEN 3 valve demonstrated comparable clinical outcomes, valve durability, and survival to surgical aortic valve replacement (SAVR) in patients with severe symptomatic aortic stenosis (AS) at low surgical risk, according to findings from the PARTNER 3 trial presented by Dr. Michael J. Mack and Dr. Martin B. Leon at TCT 2025 and simultaneously published inThe New England Journal of Medicine. The PARTNER 3 trial, conducted across multiple international centers, is the largest randomized study comparing TAVR and SAVR in low-risk patients. The study enrolled 1,000 participants (mean age 73 years, 69% males) with severe symptomatic aortic stenosis and a Society of Thoracic Surgeons (STS) score below 4%. Patients were randomized 1:1 to undergo either transfemoral TAVR using the balloon-expandable SAPIEN 3 valve or surgical bioprosthetic valve replacement. Clinical follow-up was scheduled at 30 days, six months, one year, and annually thereafter for 10 years. Echocardiographic assessments was scheduled at 30 days, 6 months, 1 to 5 yrs annually, 7 years, & 10 years. At seven years, the two prespecified primary endpoints—composite rates of all-cause death, stroke, or rehospitalization due to valve-, procedure-, or heart failure—showed no significant difference between groups. Event-free survival for death, stroke, or rehospitalization was 65.4% for TAVR and 62.8% for SAVR (HR 0.87; 95% CI, 0.70–1.08; p=0.21). Similarly, the hierarchical composite endpoint of all-cause death, disabling and non-disabling stroke, and rehospitalization days demonstrated no difference (win ratio 1.04; 95% CI, 0.84–1.30; p=0.70). All-cause mortality at seven years was 19.5% for TAVR and 16.8% for SAVR (HR 1.17; 95% CI, 0.86–1.59; p=0.31), while stroke rates were nearly identical (8.5% vs. 8.1%; HR 1.00; p=0.99). Rehospitalization related to valve, procedure, or heart failure occurred in 20.6% of TAVR and 23.5% of SAVR patients (HR 0.82; 95% CI, 0.62–1.10; p=0.18). Valve function and durability remained excellent and comparable. Mean aortic valve gradients and valve areas were stable from one to seven years in both groups, with no significant difference at follow-up. The incidence of bioprosthetic valve failure was low (6.9% for TAVR vs. 7.3% for SAVR; HR 0.93; 95% CI, 0.56–1.54; p=0.78). Rates of aortic valve reintervention were also similar (6.7% vs. 6.0%; p=0.72). TAVR was associated with lower rates of new-onset atrial fibrillation (17.7% vs. 43.5%; p<0.0001), while surgical patients had fewer instances of clinical valve thrombosis (0.5% vs. 2.8%; p<0.01). Both groups had minimal rates of endocarditis (≈3%) and low serious bleeding rates. Quality-of-life improvements measured by the Kansas City Cardiomyopathy Questionnaire (KCCQ) were large and sustained through seven years, with mean scores exceeding 85 in both arms. Investigators emphasized that these long-term findings demonstrate durable outcomes and comparable valve performance between TAVR and surgery. The PARTNER 3 trial reinforces the long-term viability of SAPIEN 3 TAVR in low-risk patients with severe aortic stenosis and underscores the importance of continued surveillance to assess valve durability as the population of younger, lower surgical risk patients receiving transcatheter therapy expands. Reference:Michael J. Mack et al., Seven year Outcomes of the PARTNER 3 Low-risk Trial, TCT Conference 2025, San Francisco. https://www.tctconference.com/ About the study presenter:Dr. Mack earned his medical degree from the St. Louis University after which he completed an Internal Medicine residency at the University of Minnesota. Dr. Mack then completed his General Surgery and Thoracic Surgery residencies at the University of Texas Southwestern. Dr. Mack is a pioneer in the field of cardiothoracic surgery and a world-renowned physician. He is the Medical Director of Cardiothoracic Surgery for Baylor Scott & White Health and the Chairman of BSW The Heart Hospital. He has performed more than 7,000 cardiac surgeries, with 3,000 involving heart valve procedures. Dr. Mack has served in multiple leadership roles including being a Board of Trustees on the American College of Cardiology Foundation, President of the Society of Thoracic Surgeons, President of the Thoracic Surgery Foundation for Research and Education, and President of the Southern Thoracic Surgical Association.
Disclaimer: This website is designed for healthcare professionals and serves solely for informational purposes.
The content provided should not be interpreted as medical advice, diagnosis, treatment recommendations, prescriptions, or endorsements of specific medical practices. It is not a replacement for professional medical consultation or the expertise of a licensed healthcare provider.
Given the ever-evolving nature of medical science, we strive to keep our information accurate and up to date. However, we do not guarantee the completeness or accuracy of the content.
If you come across any inconsistencies, please reach out to us at
admin@doctornewsdaily.com.
We do not support or endorse medical opinions, treatments, or recommendations that contradict the advice of qualified healthcare professionals.
By using this website, you agree to our
Terms of Use,
Privacy Policy, and
Advertisement Policy.
For further details, please review our
Full Disclaimer.
Tags:
Recent News
mRNA COVID vaccines may improve survival rates in...
- 29 October, 2025
Scientists discover unexpected link between gray h...
- 29 October, 2025
Eating peanuts during pregnancy may affect how a c...
- 29 October, 2025
New Drug-Eluting Balloon Matches Standard Stents i...
- 29 October, 2025
Daily Newsletter
Get all the top stories from Blogs to keep track.


0 Comments
Post a comment
No comments yet. Be the first to comment!