EVOQUE Transcatheter Tricuspid Valve Replacement (TTVR) Demonstrates Favorable Real-World Outcomes: Early U.S. Experience Confirms Safety, Effectiveness, and Quality-of-Life Gains
- byDoctor News Daily Team
- 28 October, 2025
- 0 Comments
- 0 Mins
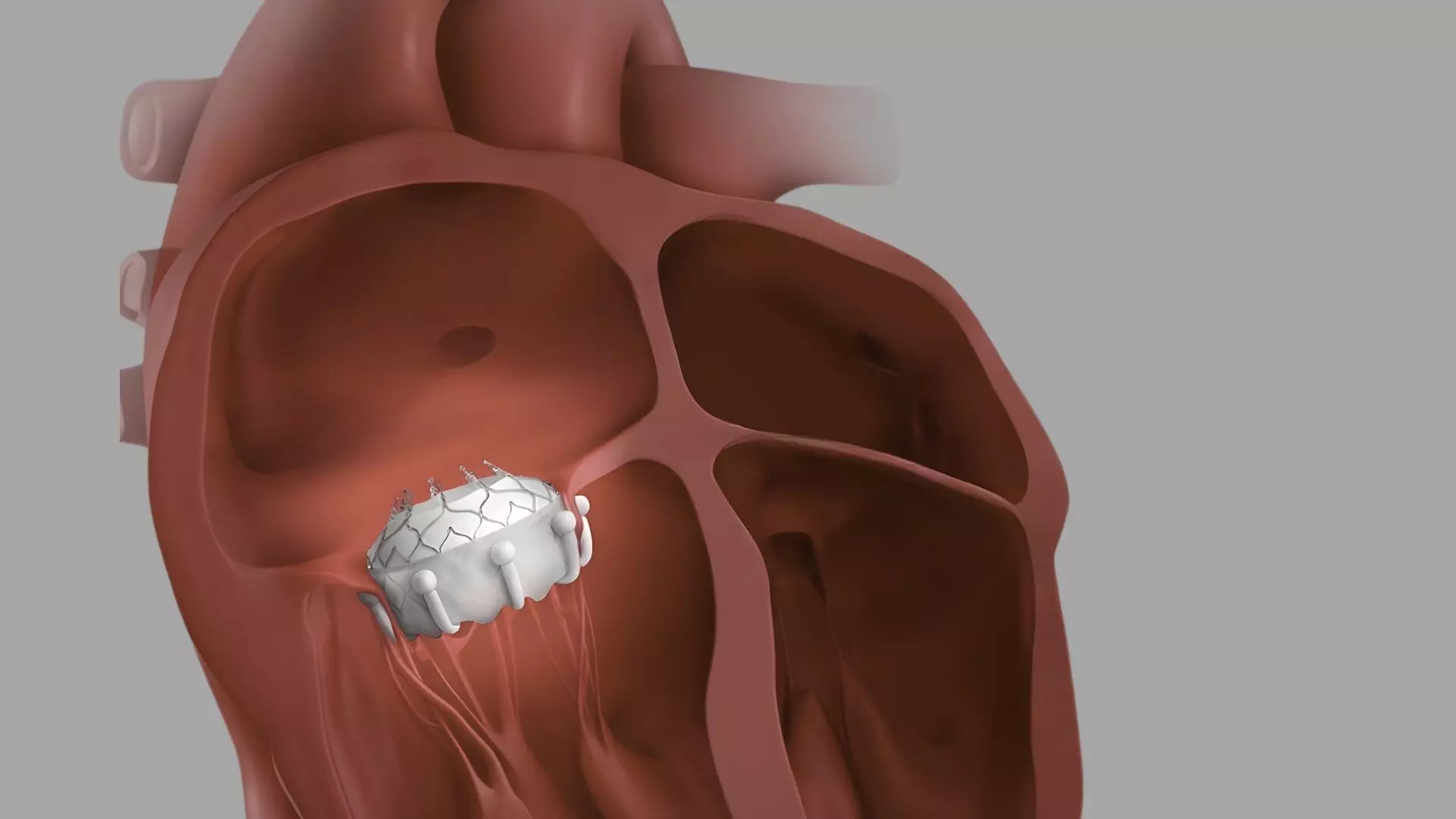
This news is covered by the Bureau present at the TCT Conference 2025, being held in San Francisco, USA. Transcatheter tricuspid valve replacement (TTVR) with the EVOQUE system achieved near-complete elimination of tricuspid regurgitation (TR), significant improvements in functional capacity and quality of life, and low complication rates at 30 days, according to the first U.S. real-world data from the STS/ACC TVT Registry. The results were presented by Dr Rahul Sharma of Stanford University at TCT 2025. The registry analysis evaluated early commercial outcomes of the EVOQUE system, a transcatheter tricuspid valve replacement device designed for patients with severe, symptomatic TR who are at high surgical risk. Data were collected from 1,034 patients treated at 82 sites across the United States between February 2024 and March 2025. The analysis was performed by the Smith Center for Outcomes Research at Beth Israel Deaconess Medical Center. Tricuspid regurgitation is associated with right heart failure, frequent hospitalizations, and increased mortality. The EVOQUE system features a self-expanding nitinol frame and intra-annular sealing skirt, allowing it to conform to the native valve anatomy and effectively eliminate regurgitation. This registry provides the first large-scale, real-world assessment of the device’s safety and efficacy following U.S. commercial introduction. Procedural success was high, with most cases completed via transfemoral access. Median hospital stay was two days, and nearly all patients were discharged home. In-hospital all-cause mortality was 2.3%, rising slightly to 3.1% at 30 days. Cardiovascular mortality was 2.0%, while major or life-threatening bleeding occurred in 1.3%, and major vascular complications in 1.4%. The rate of new pacemaker implantation was 9.9%, and tricuspid valve reintervention occurred in only 0.5% of patients. Thirty-day heart failure hospitalization was reported in 3.1%. Echocardiographic outcomes showed excellent procedural success, with more than 97% of patients achieving mild or less residual TR at 30 days. Functional capacity and quality of life improved markedly, reflected by enhanced New York Heart Association (NYHA) class and Kansas City Cardiomyopathy Questionnaire (KCCQ) scores. These benefits were consistent across subgroups, including those with and without prior pacemakers. Notably, bleeding and pacemaker rates were lower than previously seen in randomized studies, suggesting improved procedural techniques and operator experience during real-world adoption. Investigators acknowledged limitations such as the absence of core laboratory adjudication for echocardiographic data and reliance on site-reported events without centralized clinical events committee review. As an observational registry, findings may be influenced by incomplete data reporting. In summary, the early U.S. commercial experience from the STS/ACC TVT Registry demonstrates that the EVOQUE system provides a safe, effective, and durable transcatheter option for patients with severe tricuspid regurgitation. The strong 30-day outcomes reinforce its role as a transformative therapy in managing right-sided heart valve disease, with broad applicability across U.S. centers. Reference:Rahul Sharma et al.,Evaluating Real-World Outcomes of TTVR - Early commercial experience from the STS/ACC TVT Registry with the EVOQUE System, TCT Conference 2025, San Francisco.https://www.tctconference.com/
Disclaimer: This website is designed for healthcare professionals and serves solely for informational purposes.
The content provided should not be interpreted as medical advice, diagnosis, treatment recommendations, prescriptions, or endorsements of specific medical practices. It is not a replacement for professional medical consultation or the expertise of a licensed healthcare provider.
Given the ever-evolving nature of medical science, we strive to keep our information accurate and up to date. However, we do not guarantee the completeness or accuracy of the content.
If you come across any inconsistencies, please reach out to us at
admin@doctornewsdaily.com.
We do not support or endorse medical opinions, treatments, or recommendations that contradict the advice of qualified healthcare professionals.
By using this website, you agree to our
Terms of Use,
Privacy Policy, and
Advertisement Policy.
For further details, please review our
Full Disclaimer.
Recent News
Merck Keytruda wins European Commission nod for lo...
- 30 October, 2025
UP NEET 2025 round 3 allotment results postponed
- 30 October, 2025
Achin Gupta to succeed Umang Vohra as Cipla MD, GC...
- 30 October, 2025
Mumbai shocker: KEM Hospital doctor stabbed by col...
- 30 October, 2025
Daily Newsletter
Get all the top stories from Blogs to keep track.


0 Comments
Post a comment
No comments yet. Be the first to comment!