Depot Medroxyprogesterone Acetate Use Linked to Higher Meningioma Risk in Women: JAMA
- byDoctor News Daily Team
- 30 September, 2025
- 0 Comments
- 0 Mins
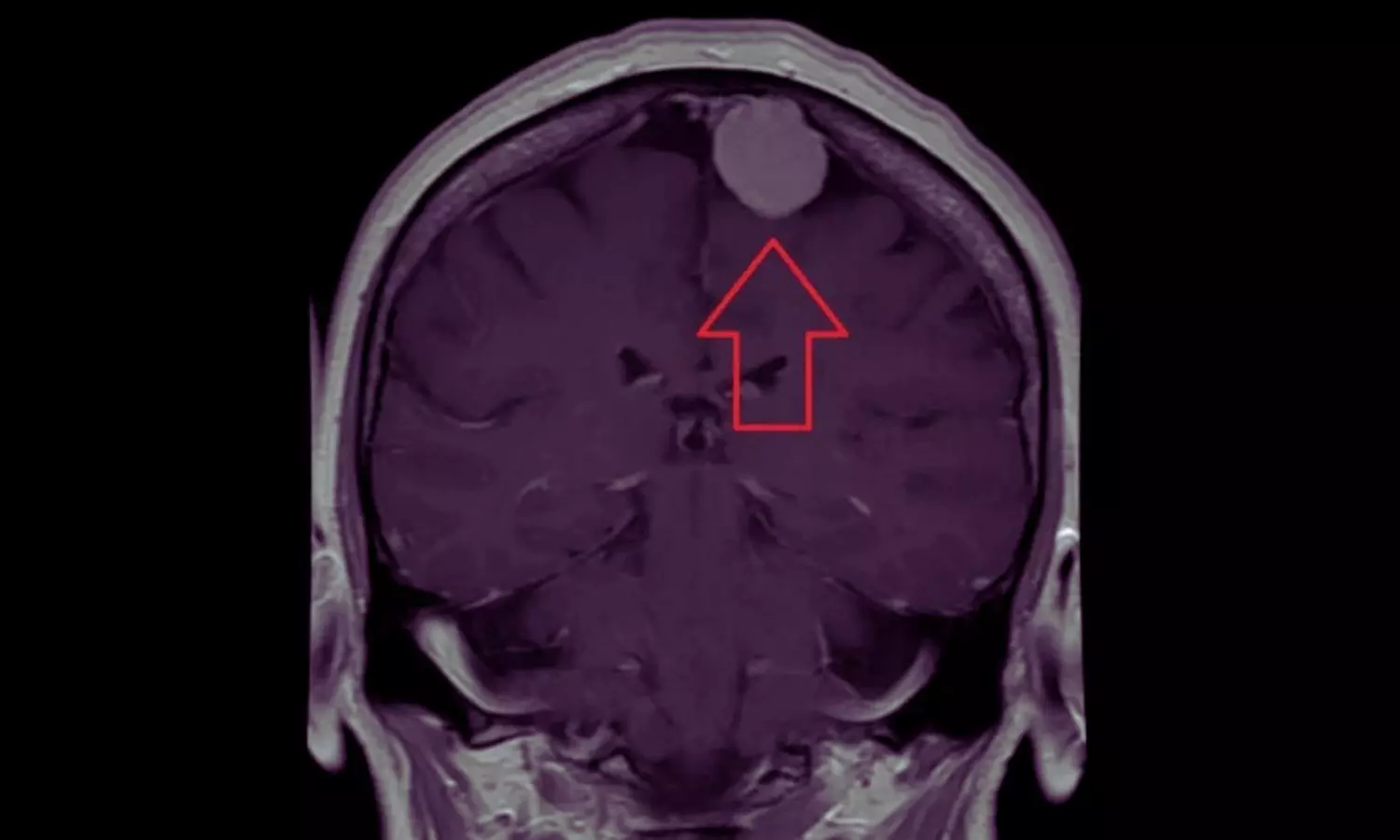
Researchers have found in a new study that women using depot medroxyprogesterone acetate had a higher risk of developing meningioma. Investigators have determined that women who used depot medroxyprogesterone acetate (DMPA) were at higher risk for the development of meningioma, especially with long-term use or when it was initiated at an older age. This finding is based on a large retrospective population-based cohort study that examined data in the TriNetX US national database from about 68 health care organizations from 2004 through 2024. The study was published inJAMA Neurologyby Tianqi X. and colleagues. The objective was to elucidate the risk of meningioma in women exposed to depot medroxyprogesterone acetate, oral medroxyprogesterone acetate, and other contraceptives like combined oral contraceptives, intrauterine devices (IUDs), progestin-only pills, and subdermal implants, versus women with no exposure to such agents. The investigation had a cohort of 10,425,438 women with a mean age of 33.4 years at entry. The final analysis in propensity-score matched the final sample of 88,667 women on depot medroxyprogesterone acetate (mean age 26.2 years) against a matched control group. The main outcome was meningioma diagnosis identified through routine diagnostic codes. Relative risks (RRs) and number needed to harm (NNH) were estimated to evaluate clinical significance. The results showed women who were administered depot medroxyprogesterone acetate to have a relative risk of 2.43 (95% CI, 1.77–3.33) for meningioma versus controls. This elevated risk was experienced mainly in those patients with greater than 4 years of treatment or initiators who began therapy at ages greater than 31 years. Oral medroxyprogesterone acetate had a less dramatic but statistically significant risk (RR 1.18; 95% CI, 1.10–1.27). No enhanced risk of meningioma was recognized in combined oral contraceptives, IUDs, progestin-only pills, or subdermal implants. The number needed to harm (NNH) was calculated to be 1,152 patients for depot medroxyprogesterone acetate and 3,020 patients for oral medroxyprogesterone acetate, showing that the absolute clinical risk is still quite low even with the relative increase in risk. This big US-based study gives strong evidence that depot medroxyprogesterone acetate is related to an increased risk of meningioma, especially with long duration and advanced age at start. Nevertheless, the great number needed to harm implies that the absolute clinical risk is still low.
Disclaimer: This website is designed for healthcare professionals and serves solely for informational purposes.
The content provided should not be interpreted as medical advice, diagnosis, treatment recommendations, prescriptions, or endorsements of specific medical practices. It is not a replacement for professional medical consultation or the expertise of a licensed healthcare provider.
Given the ever-evolving nature of medical science, we strive to keep our information accurate and up to date. However, we do not guarantee the completeness or accuracy of the content.
If you come across any inconsistencies, please reach out to us at
admin@doctornewsdaily.com.
We do not support or endorse medical opinions, treatments, or recommendations that contradict the advice of qualified healthcare professionals.
By using this website, you agree to our
Terms of Use,
Privacy Policy, and
Advertisement Policy.
For further details, please review our
Full Disclaimer.
Recent News
Air Pollution May Slow Infant Brain Development: S...
- 23 October, 2025
AI Outperforms Traditional Tools in Predicting Hea...
- 23 October, 2025
Nearly Half of Acute Pancreatitis Patients Develop...
- 23 October, 2025
Can Fat You Can’t See Put You at Risk for Stroke a...
- 23 October, 2025
Daily Newsletter
Get all the top stories from Blogs to keep track.


0 Comments
Post a comment
No comments yet. Be the first to comment!