Bioadapter, Bioresorbable Polymer Coated Coronary Implant Found Better than DES at 2 years: INFINITY SWEDEHEART Trial, TCT 2025
- byDoctor News Daily Team
- 31 October, 2025
- 0 Comments
- 0 Mins
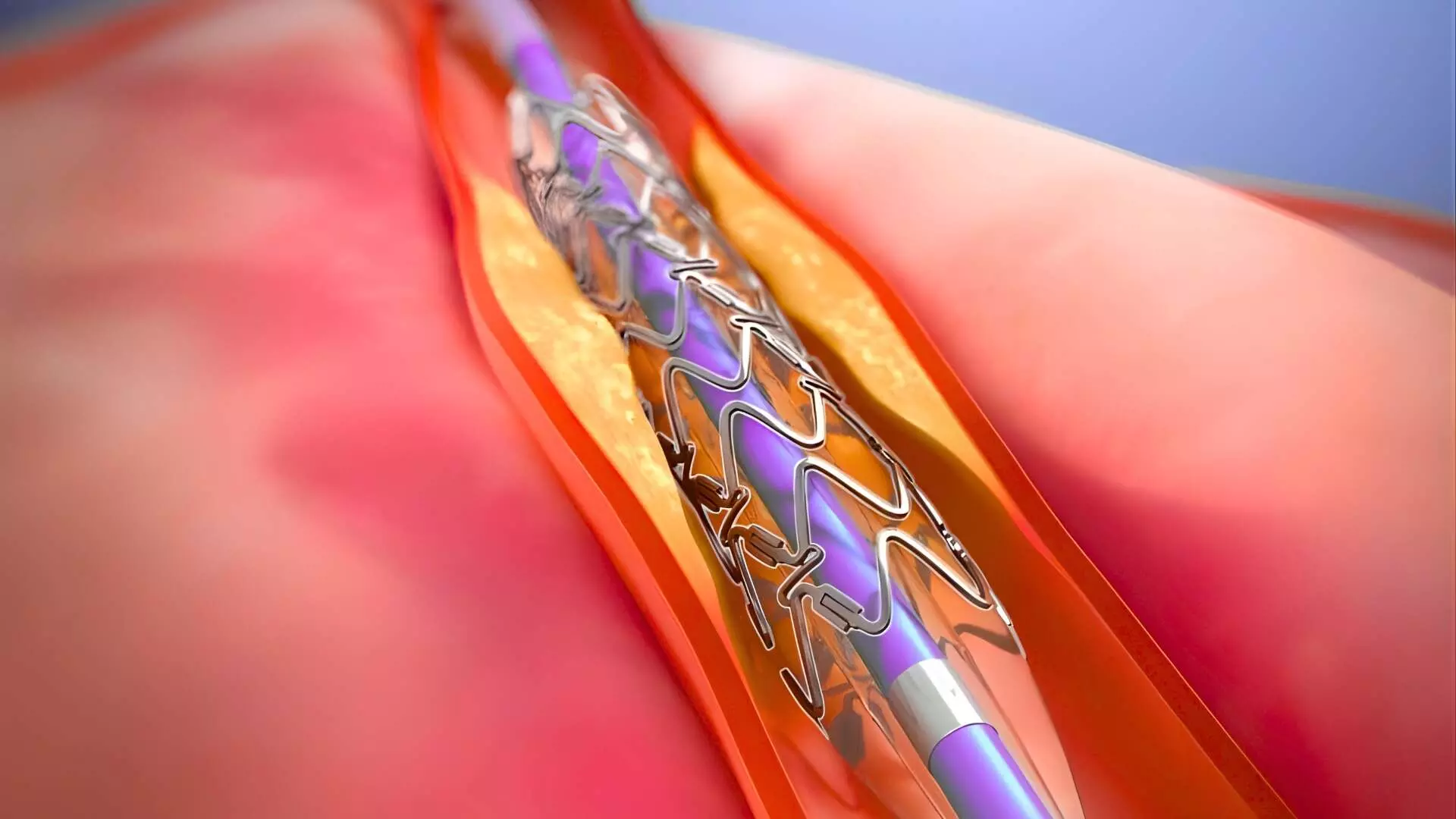
This news is covered by the Bureau present at the TCT Conference 2025, being held in San Francisco, USA. At two years, the DynamX Sirolimus-Eluting Coronary Bioadaptor demonstrated a 48% reduction in target lesion failure (TLF) compared with the Resolute Onyx drug-eluting stent (DES) after the six-month landmark period, marking the first significant long-term clinical benefit shown by a novel coronary implant over a contemporary DES. The findings from the INFINITY-SWEDEHEART randomized controlled trial were presented by Dr. David Erlinge of Lund University on behalf of the study investigators at TCT 2025. The INFINITY-SWEDEHEART trial is a large, multicenter randomized study comparing the DynamX bioadaptor (Elixir Medical, California) with the Resolute Onyx DES in a representative all-comers population, including a majority with acute coronary syndrome (ACS) and complex coronary anatomy. The trial enrolled 2,400 patients across 20 Swedish centers, with 100% follow-up completion at 24 months. Patients were randomized 1:1 to the bioadaptor (n=1,201) or the DES (n=1,198). The DynamX bioadaptor is a cobalt-chromium (71 µm) sirolimus-eluting implant temporarily connected by a bioresorbable polymer coating. After six months, the polymer unlocks, allowing the helical strands to separate and restore vessel pulsatility and natural motion—addressing long-term complications associated with rigid metallic stents. The trial was powered to demonstrate non-inferiority for TLF at one year and superiority beyond six months for TLF, target vessel failure (TVF), and TLF in ACS subgroups. Baseline characteristics were well balanced between groups (mean age 68 years; 24% women; 77% presenting with ACS). At one year, the bioadaptor met its primary non-inferiority endpoint, with TLF rates of 2.35% versus 2.77% for DES (difference –0.41%; 95% CI –1.94 to 1.11; p<0.0001 for non-inferiority). However, between six months and two years, landmark analyses revealed statistically significant superiority of the bioadaptor, with a 48% relative reduction in TLF (HR 0.52; 95% CI 0.29–0.93; p=0.027) and lower TVF (p=0.048). In patients presenting with ACS, the benefit was even greater, with a 45% reduction in TLF (p=0.0175). The reduction in TLF was driven consistently by all components—lower cardiovascular death, target vessel myocardial infarction, and ischemia-driven target lesion revascularization. Definite or probable stent thrombosis was also significantly lower with the bioadaptor (0.43% vs. 0.51%; HR 0.52, p=0.027). The trial also showed that bioadaptor performance remained robust in high-risk groups, including ACS and complex lesions such as long, calcified, and bifurcated arteries. No increase in procedural complications or early safety concerns was observed. The study concluded that the DynamX bioadaptor represents a paradigm shift in coronary intervention by combining acute safety of DES with long-term physiological vessel restoration. The INFINITY-SWEDEHEART trial thus provides the first randomized evidence of a coronary implant showing late clinical benefit over contemporary DES, a key milestone in the evolution of coronary stent technology. Reference:David Erlinge et al., Long Term Clinical Outcomes in Patients Treated with the Bioadaptor Compared to a Contemporary Drug Eluting Stent from the INFINITY-SWEDEHEART RCT: Through 2-Year Follow-Up, TCT Conference 2025, San Francisco. https://www.tctconference.com/ About the Study Presenter:David Erlinge received an MD at Lund University, Sweden 1990 followed by a PhD at the same university. He has been a visiting research fellow at Cornell Univ. Med. Coll., New York, NY. In 2006 he received the The Lars Werkö distinguished research fellowship and in 2008 he was appointed Professor in Cardiology at Lund University. Dr Erlinge has written more than 300 original articles.
Disclaimer: This website is designed for healthcare professionals and serves solely for informational purposes.
The content provided should not be interpreted as medical advice, diagnosis, treatment recommendations, prescriptions, or endorsements of specific medical practices. It is not a replacement for professional medical consultation or the expertise of a licensed healthcare provider.
Given the ever-evolving nature of medical science, we strive to keep our information accurate and up to date. However, we do not guarantee the completeness or accuracy of the content.
If you come across any inconsistencies, please reach out to us at
admin@doctornewsdaily.com.
We do not support or endorse medical opinions, treatments, or recommendations that contradict the advice of qualified healthcare professionals.
By using this website, you agree to our
Terms of Use,
Privacy Policy, and
Advertisement Policy.
For further details, please review our
Full Disclaimer.
Recent News
Scientists discover a hidden antibiotic 100 times...
- 31 October, 2025
Gut microbes may convert fiber into extra calories...
- 31 October, 2025
Who's eligible for faculty appointment? NMC clarif...
- 31 October, 2025
AMU JN Medical College launches code blue emergenc...
- 31 October, 2025
Daily Newsletter
Get all the top stories from Blogs to keep track.


0 Comments
Post a comment
No comments yet. Be the first to comment!