Baricitinib not linked to increased VTE Risk in Alopecia Areata Patients: Study
- byDoctor News Daily Team
- 09 September, 2025
- 0 Comments
- 0 Mins
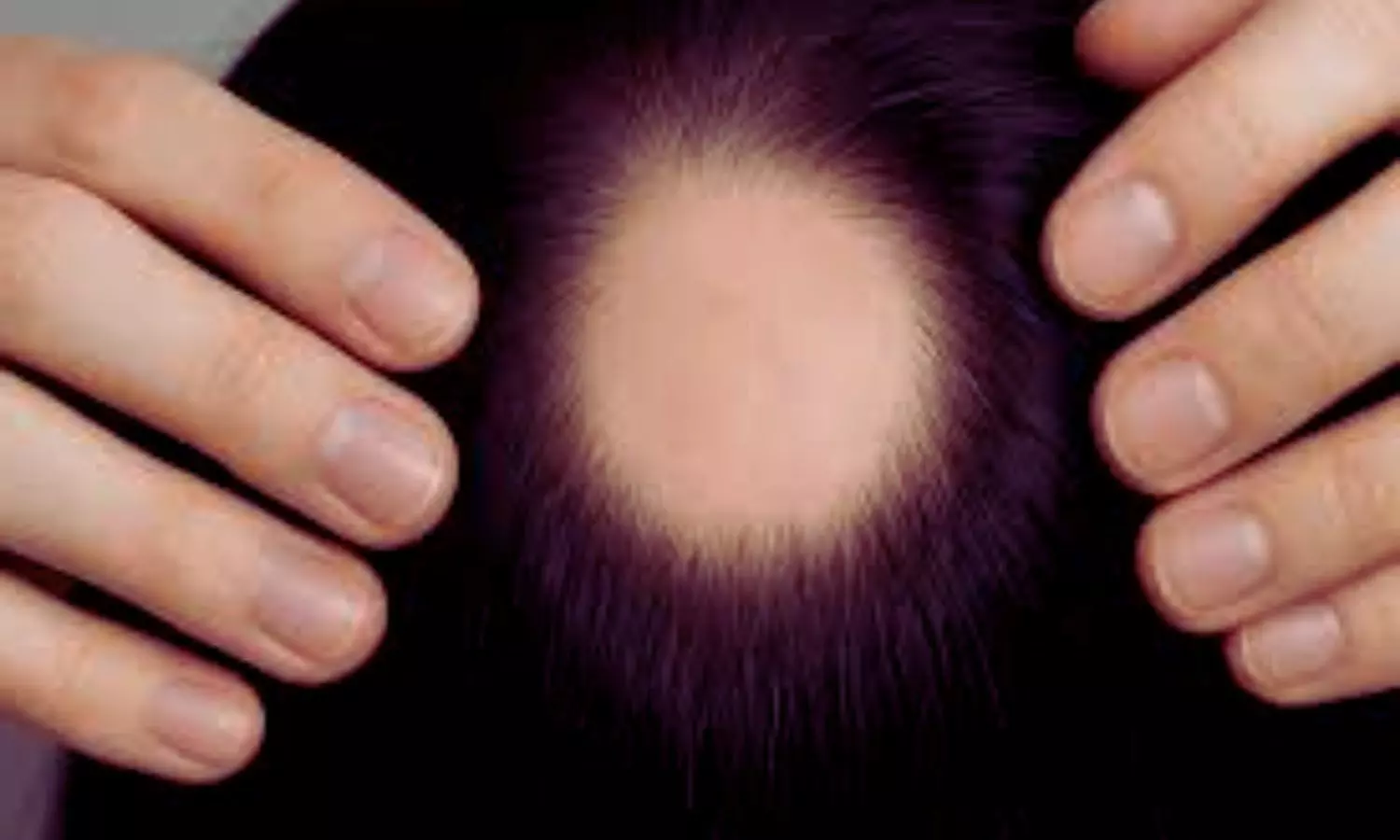
A new study published in theInternational Journal of Dermatologyrevealed that alopecia areata patients treated with baricitinib had no reported thrombotic events over 6 months, indicating no increased risk of venous thromboembolism (VTE) in this population. Based on post-marketing data from patients with rheumatoid arthritis (RA) receiving tofacitinib, the European Medicines Agency (EMA) released an advisory in January 2023 on the risk of VTE associated with Janus kinase inhibitors (JAKi) in chronic inflammatory disorders. Given that RA is a recognized independent risk factor for VTE, this might be biased. Additionally, the effects of JAKi differ due to differences in enzyme selectivity. In this investigation, VTE risk factors were examined in a cohort of AA patients who were eligible for baricitinib therapy. Additionally, any changes in laboratory VTE markers following 6 months of baricitinib treatment were evaluated. The patients with moderate-to-severe AA who were ≥ 18 years old, suitable for therapy with baricitinib 4 mg, and who presented to the department consecutively between July 2023 and July 2024 were included in a prospective observational research. During the screening appointment, baseline clinical and demographic information was gathered. Patients with major (a prior VTE episode) or minor (smoking status, active malignancies, family history of VTE, prior thrombophlebitis, recent trauma or fractures, prolonged immobilization, major recent surgeries, use of estrogen-progestin contraceptives, and recurrent miscarriages) clinical risk factors for VTE were also identified through a comprehensive medical history check. Additionally, baseline and 6-month therapy levels were assessed for antiphospholipid antibodies (lupus anticoagulant, anti-cardiolipin, and anti-β2 glycoprotein I), protein C, antithrombin III, homocysteine, protein S, and factor VIII. After being evaluated for baricitinib therapy eligibility, 47 individuals with moderate-to-severe AA were included to the trial. Based on the previously published criteria, none of the screened patients showed a contraindication to the therapy. In terms of clinical VTE risk factors at baseline, 1 patient (2.1%) had a history of superficial thrombophlebitis, 5 women (10.6%) were taking combination estrogen-progestin contraceptives, and 8 out of 47 patients (17%) were heavy smokers. There were no significant risk factors in any of the patients. Although two patients (4.2%) had bone fractures, which are recognized clinical risk factors for VTE, there were no thrombotic events while on baricitinib. Laboratory tests conducted on 37 out of 47 patients after 6 months of continuous medication revealed no changes in the majority of patients. Overall, these results imply that the risk of experiencing adverse events from VTE might be decreased for individuals who are eligible for baricitinib therapy. Caldarola, G., Ferretti, A., Pinto, L. M., Di Gennaro, L., De Luca, E., Falco, G. M., De Simone, C., De Cristofaro, R., & Peris, K. (2025). No increased risk of thromboembolic events during 6-month treatment with baricitinib in patients with alopecia areata. International Journal of Dermatology, ijd.70051.https://doi.org/10.1111/ijd.70051
Disclaimer: This website is designed for healthcare professionals and serves solely for informational purposes.
The content provided should not be interpreted as medical advice, diagnosis, treatment recommendations, prescriptions, or endorsements of specific medical practices. It is not a replacement for professional medical consultation or the expertise of a licensed healthcare provider.
Given the ever-evolving nature of medical science, we strive to keep our information accurate and up to date. However, we do not guarantee the completeness or accuracy of the content.
If you come across any inconsistencies, please reach out to us at
admin@doctornewsdaily.com.
We do not support or endorse medical opinions, treatments, or recommendations that contradict the advice of qualified healthcare professionals.
By using this website, you agree to our
Terms of Use,
Privacy Policy, and
Advertisement Policy.
For further details, please review our
Full Disclaimer.
Recent News
Gum disease could silently cause serious brain dam...
- 03 November, 2025
Can Early-Day Fasting Significantly Boost Metaboli...
- 03 November, 2025
Delhi HC bars doctor from running medical centre d...
- 03 November, 2025
Phase III data for Gazyva/Gazyvaro show significan...
- 03 November, 2025
Daily Newsletter
Get all the top stories from Blogs to keep track.


0 Comments
Post a comment
No comments yet. Be the first to comment!